Transform retail operations with Zebra’s retail technology solutions, featuring hardware and software for improving inventory management and empowering teams.
Streamline operations with Zebra’s healthcare technology solutions, featuring hardware and software to improve staff collaboration and optimise workflows.
Enhance processes with Zebra’s manufacturing technology solutions, featuring hardware and software for automation, data analysis, and factory connectivity.
Zebra’s transportation and logistics technology solutions feature hardware and software for enhancing route planning, visibility, and automating processes.
Zebra's public sector technology solutions enhance decision-making, streamline operations, and safeguard communities with advanced software and rugged hardware.
Zebra's hospitality technology solutions equip your hotel and restaurant staff to deliver superior customer and guest service through inventory tracking and more.
Zebra's market-leading solutions and products improve customer satisfaction with a lower cost per interaction by keeping service representatives connected with colleagues, customers, management and the tools they use to satisfy customers across the supply chain.
Empower your field workers with purpose-driven mobile technology solutions to help them capture and share critical data in any environment.
Zebra's range of mobile computers equip your workforce with the devices they need from handhelds and tablets to wearables and vehicle-mounted computers.
Zebra's desktop, mobile, industrial, and portable printers for barcode labels, receipts, RFID tags and cards give you smarter ways to track and manage assets.
Zebra's 1D and 2D corded and cordless barcode scanners anticipate any scanning challenge in a variety of environments, whether retail, healthcare, T&L or manufacturing.
Zebra's extensive range of RAIN RFID readers, antennas, and printers give you consistent and accurate tracking.
Choose Zebra's reliable barcode, RFID and card supplies carefully selected to ensure high performance, print quality, durability and readability.
Zebra's location technologies provide real-time tracking for your organisation to better manage and optimise your critical assets and create more efficient workflows.
Zebra's rugged tablets and 2-in-1 laptops are thin and lightweight, yet rugged to work wherever you do on familiar and easy-to-use Windows or Android OS.
With Zebra's family of fixed industrial scanners and machine vision technologies, you can tailor your solutions to your environment and applications.
Zebra’s line of kiosks can meet any self-service or digital signage need, from checking prices and stock on an in-aisle store kiosk to fully-featured kiosks that can be deployed on the wall, counter, desktop or floor in a retail store, hotel, airport check-in gate, physician’s office, local government office and more.
Discover Zebra’s range of accessories from chargers, communication cables to cases to help you customise your mobile device for optimal efficiency.
Zebra's environmental sensors monitor temperature-sensitive products, offering data insights on environmental conditions across industry applications.
Adapt to market shifts, enhance worker productivity and secure long-term growth with AMRs. Deploy, redeploy and optimize autonomous mobile robots with ease.
Enhance frontline operations with Zebra’s AI software solutions, which optimize workflows, streamline processes, and simplify tasks for improved business outcomes.
Zebra Workcloud, enterprise software solutions boost efficiency, cut costs, improve inventory management, simplify communication and optimize resources.
Keep labour costs low, your talent happy and your organisation compliant. Create an agile operation that can navigate unexpected schedule changes and customer demand to drive sales, satisfy customers and improve your bottom line.
Drive successful enterprise collaboration with prioritized task notifications and improved communication capabilities for easier team collaboration.
Get full visibility of your inventory and automatically pinpoint leaks across all channels.
Reduce uncertainty when you anticipate market volatility. Predict, plan and stay agile to align inventory with shifting demand.
Drive down costs while driving up employee, security, and network performance with software designed to enhance Zebra's wireless infrastructure and mobile solutions.
Explore Zebra’s printer software to integrate, manage and monitor printers easily, maximising IT resources and minimising down time.
Make the most of every stage of your scanning journey from deployment to optimisation. Zebra's barcode scanner software lets you keep devices current and adapt them to your business needs for a stronger ROI across the full lifecycle.
RFID development, demonstration and production software and utilities help you build and manage your RFID deployments more efficiently.
RFID development, demonstration and production software and utilities help you build and manage your RFID deployments more efficiently.
Zebra DNA is the industry’s broadest suite of enterprise software that delivers an ideal experience for all during the entire lifetime of every Zebra device.
Advance your digital transformation and execute your strategic plans with the help of the right location and tracking technology.
Boost warehouse and manufacturing operations with Symmetry, an AMR software for fleet management of Autonomous Mobile Robots and streamlined automation workflows.
The Zebra Aurora suite of machine vision software enables users to solve their track-and-trace, vision inspection and industrial automation needs.
Zebra Aurora Focus brings a new level of simplicity to controlling enterprise-wide manufacturing and logistics automation solutions. With this powerful interface, it’s easy to set up, deploy and run Zebra’s Fixed Industrial Scanners and Machine Vision Smart Cameras, eliminating the need for different tools and reducing training and deployment time.
Aurora Imaging Library™, formerly Matrox Imaging Library, machine-vision software development kit (SDK) has a deep collection of tools for image capture, processing, analysis, annotation, display, and archiving. Code-level customisation starts here.
Aurora Design Assistant™, formerly Matrox Design Assistant, integrated development environment (IDE) is a flowchart-based platform for building machine vision applications, with templates to speed up development and bring solutions online quicker.
Designed for experienced programmers proficient in vision applications, Aurora Vision Library provides the same sophisticated functionality as our Aurora Vision Studio software but presented in programming language.
Aurora Vision Studio, an image processing software for machine & computer vision engineers, allows quick creation, integration & monitoring of powerful OEM vision applications.
Adding innovative tech is critical to your success, but it can be complex and disruptive. Professional Services help you accelerate adoption, and maximise productivity without affecting your workflows, business processes and finances.
Zebra's Managed Service delivers worry-free device management to ensure ultimate uptime for your Zebra Mobile Computers and Printers via dedicated experts.
Find ways you can contact Zebra Technologies’ Support, including Email and Chat, ask a technical question or initiate a Repair Request.
Zebra's Circular Economy Program helps you manage today’s challenges and plan for tomorrow with smart solutions that are good for your budget and the environment.
What Is a Vaccine Vial Monitor and How Does It Work?
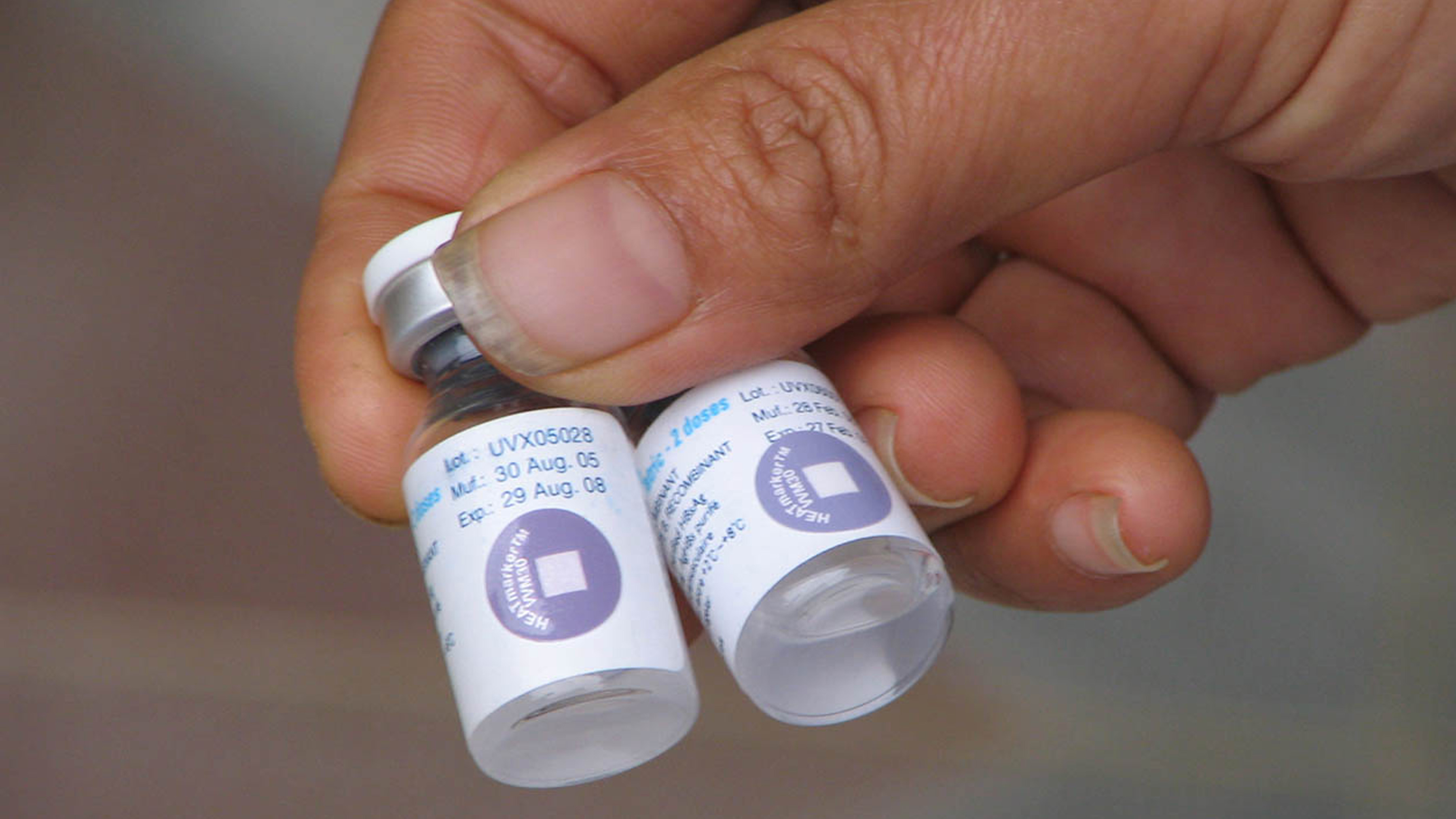
A Vaccine Vial Monitor (VVM) is a label containing a heat-sensitive material that is placed on a vaccine vial to register cumulative heat exposure over time. The VVM works by gradually changing color as it's exposed to heat. The combined effects of time and temperature cause the inner square of the VVM to darken gradually and irreversibly. The rate of color change increases with temperature. So, if a vaccine is kept in a very hot place, the VVM will darken more quickly than if the vaccine is kept in a relatively cooler place. Here's why this is important: vaccines need to be stored within a certain temperature range to remain effective. If a vaccine gets too hot, it can lose its effectiveness. This is especially a concern in areas with high temperatures or where refrigeration may not always be reliable.
The VVM is affixed to a vaccine vial by the vaccine manufacturer to allow for full-life heat monitoring. It allows healthcare professionals to administer vaccines, confident that the vaccine has not been exposed to potentially damaging heat. Even if a vaccine was exposed to an inadvertent heat excursion, the VVM provides a visual cue if the vaccine could still be used and its use can help reduce wastage and increase coverage.
Since a VVM and the vaccine move through the supply chain together, they are exposed to the same conditions as measured by Mean Kinetic Temperature (MKT). This allows the VVM to give health workers an overview of the cumulative heat exposure of the vaccine and a clear indication of when it has reached its time-temperature endpoint.
VVMs are time-temperature indicators that give visual warnings of potentially damaging cumulative heat exposures and indicate to healthcare workers whether a vaccine can or cannot be used.
How Does a VVM Work?
The inner square of the VVM is made of heat-sensitive material that is initially light in color and becomes darker when exposed to heat. The inner square is lighter in color than the outer circle prior to heat exposure and remains so until the heat exposure reaches a level that surpasses the approved stability limits of the vaccine. At the discard point or endpoint, the inner square is the same color as the outer circle. This indicates that the vial has been exposed to an unacceptable level of heat. The inner square continues to darken as heat exposure continues until it is much darker than the outer circle. If the inner square becomes as dark as or darker than the outer circle the vial must be discarded.
Understanding the Fundamentals of Vaccine Vial Monitors
Temperature is important to a vaccine vial monitor because vaccines are delicate biological products that can lose their potency if they are exposed to temperatures outside of their recommended range. Vaccine vial monitors (VVMs) are small stickers that are attached to vaccine vials to monitor whether the vaccine has been exposed to excessive heat or cold during storage, transport, or handling.
The VVMs are designed to change color based on the temperature history of the vaccine. For example, if the vaccine is exposed to temperatures above the recommended range, the VVM will change color to indicate that the vaccine may no longer be effective. This helps healthcare workers identify and discard vaccines that have been compromised, ensuring that only potent vaccines are administered to patients.
Therefore, temperature is a critical factor in maintaining the efficacy of vaccines, and VVMs play an important role in ensuring that vaccines are stored and transported at the correct temperature.
What Does a VVM Look Like?
The VVM is a circle composed of both a reference ring and a small inner square, called the active square or active surface. The VVM may be printed onto a product label, or it may be attached as an independent device onto the cap of a vaccine vial or tube, or to the neck of an ampoule. See the image below:

How to Read the VVM
VVM’s gradual and permanent color change from light to dark shows the temperature history of the vaccine from the time the VVM is affixed to the vaccine vial by the manufacturer – until the product is used. The inner square of the VVM is made of heat-sensitive material that is initially light in color and becomes darker when exposed to heat. The color change is faster at higher temperatures and slower at lower temperatures. At the endpoint, the inner square is the same color as the outer circle or darker than the outer circle.
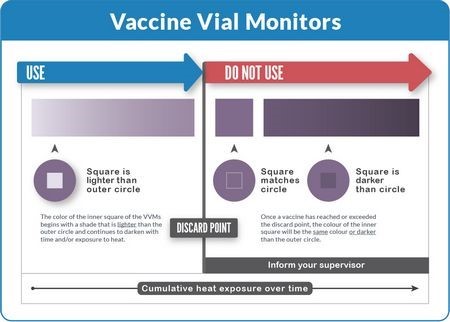
To read a VVM, compare the color of the inner square to the color of the outer circle:
Rule 1: If the inner square is lighter than the outer circle, the vaccine can be used provided that the expiry date has not passed.
Rule 2: If the inner square is the same color or darker than the outer circle, the vaccine must not be used.
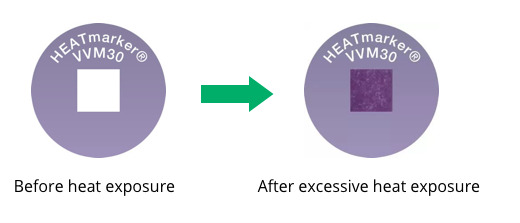
What Does a VVM Look Like As It Changes Color?
The graphic below shows the changing appearance of the VVM as it is exposed to heat over time:
How Are VVMs Measured?
To measure VVMs, place the VVMs with the release liner still attached to white card stock, then measure the Optical Density (OD) of the active indicator (I) and reference ring (R) using an X-Rite 500 series spectrodensitometer or equivalent. Measure the reference ring by taking one measurement from the bottom of the ring and a second measurement from another point 90° away. Measure the active indicator by taking two measurements from different points within the active square. To determine the optical density difference, also called R-I, subtract the average I value from the average R-value.
How Should VVMs Be stored?
Before being applied to vaccines, VVMs must be stored at temperatures < -24°C and kept away from light and other sources of radiation. Once applied to vaccines, storage conditions are based on the vaccine’s storage requirements.
What Are the Benefits of Vaccine Vial Monitors?
VVMs help to expand the reach of immunization programs to remote populations and to minimize the risk of people receiving potentially ineffective, heat-damaged vaccines along with increasing the efficiency of immunization programs worldwide.
The VVM is a critical component of vaccine manufacturers' ability to supply life-saving vaccines in support of global vaccination programs. Through a simple, predictable, reliable, and visual color change, health workers know at a glance whether a vaccine has been exposed to potentially damaging heat. Irreversible time-temperature indicators can play a vital role in the success of vaccination programs worldwide.
What Is a Vaccine Vial Monitor (VVM) Used For?
Here's how VVMs are used in different settings:
1. Vaccine storage: VVMs can be affixed to vaccine vials during storage to monitor the temperature of the vaccine. When the vaccine is removed from storage, the healthcare worker can check the VVM to ensure that the vaccine has not been exposed to temperatures outside of the recommended range.
2. Vaccine transport: VVMs can be used during vaccine transport to monitor the temperature of the vaccines. The VVMs can provide an indication of whether the vaccines have been exposed to excessive heat or cold during transport, enabling healthcare workers to identify potential breaches in temperature control.
3. Vaccine administration: VVMs can be checked by healthcare workers before vaccine administration to ensure that the vaccine is still potent. If the VVM shows that the vaccine has been compromised due to temperature fluctuations, the healthcare worker can discard the vaccine and use an alternative one to ensure that the patient receives a potent vaccine.
In day-to-day vaccine management, VVMs provide critical information about the temperature history of vaccines, enabling healthcare workers to ensure that vaccines are properly maintained and effective. By helping to prevent vaccine wastage and maintain vaccine potency, VVMs play an essential role in ensuring that communities have access to life-saving immunizations.
Why Is VVM Important in Healthcare?
Vaccine vial monitors (VVMs) give important information to health workers regarding the temperature of a vaccine. The VVM provides a visual indication of whether the vaccine has been exposed to excessive heat during storage, transport, or handling, which can affect its potency.
Specifically, VVMs provide insights to health workers such as:
1. Vaccine potency status: The color of the VVM indicates whether the vaccine is still potent or has been compromised due to temperature fluctuations. If the VVM shows that the vaccine has been exposed to excessive heat or cold, health workers can discard the vaccine and use an alternative one to ensure the patient receives a potent vaccine.
2. Temperature threshold breaches: The VVM indicates when the temperature of the vaccine has gone above or below the recommended range, indicating potential breaches in temperature control during storage, transport, or handling. This information can help identify areas in the vaccine supply chain that require improvements to maintain the cold chain and prevent temperature excursions.
Overall, VVMs provide critical information to health workers about the temperature history of a vaccine, which helps ensure that potent vaccines are administered to patients and that the vaccine supply chain is properly maintained to preserve vaccine efficacy.
What Are the Advantages of Using VVM Labels?
VVM labels can improve efficiency by providing the following:
1. Simple visual indication: The VVM label provides a simple visual indication of whether the vaccine has been exposed to excessive heat or cold. The label changes color based on the temperature history of the vaccine, making it easy for healthcare workers to identify compromised vaccines at a glance.
2. No external equipment needed: VVM labels do not require any external equipment or power supply to function. They are a passive technology that is self-contained within the label itself, making them easy to use and integrate into existing vaccine management systems.
3. Long-lasting: VVM labels have a long shelf life, which means they can potentially remain affixed to vaccine vials for a period of time without losing their effectiveness. This helps reduce the need for frequent label replacements, reducing overall costs and increasing efficiency.
4. Global standardization: VVM labels are a globally standardized technology, which means that they are widely recognized and accepted by healthcare workers, regulatory agencies, and vaccine manufacturers worldwide. This standardization helps ensure that VVM labels are used consistently and correctly, reducing errors and improving efficiency.
In today's fast and connected world, VVM labels can help provide vital information and ensure that vaccines are safe and effective when administered to patients.
Explore Zebra's full range of Vaccine Vial Monitor Solutions
Legal Terms of Use Privacy Policy Supply Chain Transparency
ZEBRA and the stylized Zebra head are trademarks of Zebra Technologies Corp., registered in many jurisdictions worldwide. All other trademarks are the property of their respective owners. ©2025 Zebra Technologies Corp. and/or its affiliates.

